Leveraging genome-wide datasets to quantify the functional role of the anti-Shine–Dalgarno sequence in regulating translation efficiency
Abstract
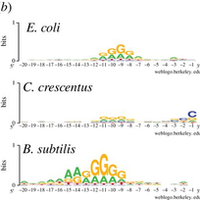
Studies dating back to the 1970s established that sequence complementarity between the anti-Shine–Dalgarno (aSD) sequence on prokaryotic ribosomes and the 5' untranslated region of mRNAs helps to facilitate translation initiation. The optimal location of aSD sequence binding relative to the start codon, the full extents of the aSD sequence and the functional form of the relationship between aSD sequence complementarity and translation efficiency have not been fully resolved. Here, we investigate these relationships by leveraging the sequence diversity of endogenous genes and recently available genome-wide estimates of translation efficiency. We show that—after accounting for predicted mRNA structure—aSD sequence complementarity increases the translation of endogenous mRNAs by roughly 50%. Further, we observe that this relationship is nonlinear, with translation efficiency maximized for mRNAs with intermediate levels of aSD sequence complementarity. The mechanistic insights that we observe are highly robust: we find nearly identical results in multiple datasets spanning three distantly related bacteria. Further, we verify our main conclusions by re-analysing a controlled experimental dataset.